What Must Happen to Ensure Successful Cell Division
The five phases of mitosis and cell division tightly coordinate the movements of hundreds of proteins. How did early biologists unravel this complex dance of chromosomes?
Perhaps the nigh amazing thing about mitosis is its precision, a feature that has intrigued biologists since Walther Flemming offset described chromosomes in the late 1800s (Paweletz, 2001). Although Flemming was able to correctly deduce the sequence of events in mitosis, this sequence could not exist experimentally verified for several decades, until advances in light microscopy made information technology possible to observe chromosome movements in living cells. Researchers now know that mitosis is a highly regulated process involving hundreds of different cellular proteins. The dynamic nature of mitosis is best appreciated when this process is viewed in living cells.
Mitosis Occupies a Portion of the Cell Cycle

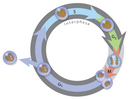
In his pioneering studies of mitosis, Flemming noted that the nuclear material, which he named "chromatin" for its power to take up stains, did not have the same advent in all cells. (We still use the discussion "chromatin" today, albeit in a more biochemical sense to refer to complexes of nuclear Deoxyribonucleic acid and protein.) Specifically, in some cells, chromatin appeared as an baggy network, although in other cells, it appeared as threadlike bodies that Flemming named "mitosen." Based on his observations, Flemming had the insight to propose that chromatin could undergo reversible transformations in cells. Today, scientists know that Flemming had successfully distinguished chromosomes in the interphase portion of the cell cycle from chromosomes undergoing mitosis, or the portion of the cell bicycle during which the nucleus divides (Effigy 1). With very few exceptions, mitosis occupies a much smaller fraction of the cell bike than interphase.
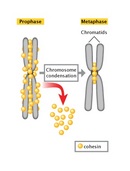
The difference in DNA compaction betwixt interphase and mitosis is dramatic. A precise approximate of the deviation is non possible, but during interphase, chromatin may exist hundreds or even thousands of times less condensed than information technology is during mitosis. For this reason, the enzyme complexes that copy Dna take the greatest access to chromosomal Deoxyribonucleic acid during interphase, at which time the vast majority of gene transcription occurs. In addition, chromosomal DNA is duplicated during a subportion of interphase known equally the Due south, or synthesis, phase. As the two daughter DNA strands are produced from the chromosomal Dna during S stage, these daughter strands recruit additional histones and other proteins to class the structures known equally sis chromatids (Figure 2). The sister chromatids, in plow, get "glued" together by a protein complex named cohesin. Cohesin is a member of the SMC, or structural maintenance of chromosomes, family unit of proteins. SMC proteins are Deoxyribonucleic acid-binding proteins that affect chromosome architectures; indeed, cells that lack SMC proteins show a variety of defects in chromosome stability or chromosome beliefs. Current data suggest that cohesin complexes may literally form circles that encompass the ii sister chromatids (Hirano, 2002; Hagstrom & Meyer, 2003). At the end of Southward phase, cells are able to sense whether their Dna has been successfully copied, using a complicated set of checkpoint controls that are nevertheless not fully understood. For the nearly part, just cells that have successfully copied their Dna volition keep into mitosis.
Chromatin Is Extensively Condensed as Cells Enter Mitosis

The about obvious difference betwixt interphase and mitosis involves the appearance of a jail cell's chromosomes. During interphase, private chromosomes are not visible, and the chromatin appears diffuse and unorganized. Recent research suggests, even so, that this is an oversimplification and that chromosomes may actually occupy specific territories within the nucleus (Cremer & Cremer, 2001). In any case, as mitosis begins, a remarkable condensation process takes place, mediated in part by another member of the SMC family unit, condensin (Hirano, 2002; Hagstrom & Meyer, 2003). Like cohesin, condensin is an elongated complex of several proteins that binds and encircles DNA. In contrast to cohesin, which binds two sister chromatids together, condensin is thought to demark a single chromatid at multiple spots, twisting the chromatin into a variety of coils and loops (Figure 3).
The Mitotic Spindle Aids in Chromosome Separation

During mitosis, chromosomes become fastened to the structure known as the mitotic spindle. In the late 1800s, Theodor Boveri created the earliest detailed drawings of the spindle based on his observations of cell division in early Ascaris embryos (Effigy four; Satzinger, 2008). Boveri'south drawings, which are amazingly accurate, testify chromosomes attached to a bipolar network of fibers. Boveri observed that the spindle fibers radiate from structures at each pole that nosotros now recognize as centrosomes, and he also noted that each centrosome contains two small, rodlike bodies, which are now known as centrioles. Boveri observed that the centrioles duplicate before the chromosomes become visible and that the two pairs of centrioles move to separate poles before the spindle assembles. We now know that centrioles duplicate during Due south phase, although many details of this duplication procedure are notwithstanding under investigation.

The limerick of the spindle fibers remained unknown until the 1960s, when tubulin was discovered and techniques were adult for visualizing spindles using electron microscopes (Mitchison & Salmon, 2001). Information technology is now well-established that spindles are bipolar arrays of microtubules composed of tubulin (Effigy v) and that the centrosomes nucleate the growth of the spindle microtubules. During mitosis, many of the spindle fibers adhere to chromosomes at their kinetochores (Effigy 6), which are specialized structures in the most constricted regions of the chromosomes. The length of these kinetochore-fastened microtubules then decreases during mitosis, pulling sister chromatids to opposite poles of the spindle. Other spindle fibers exercise not attach to chromosomes, but instead form a scaffold that provides mechanical force to separate the daughter nuclei at the end of mitosis.
Mitosis Is Divided into Well-Defined Phases

From his many detailed drawings of mitosen, Walther Flemming correctly deduced, simply could not prove, the sequence of chromosome movements during mitosis (Figure 7). Flemming divided mitosis into two broad parts: a progressive phase, during which the chromosomes condensed and aligned at the eye of the spindle, and a regressive phase, during which the sister chromatids separated. Our modern agreement of mitosis has benefited from advances in light microscopy that have allowed investigators to follow the process of mitosis in living cells. Such alive cell imaging non only confirms Flemming's observations, but it likewise reveals an extremely dynamic procedure that can only be partially appreciated in all the same images.
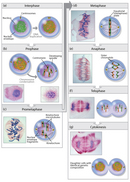
Today, mitosis is understood to involve 5 phases, based on the concrete state of the chromosomes and spindle. These phases are prophase, prometaphase, metaphase, anaphase, and telophase. Cytokinesis is the final physical cell division that follows telophase, and is therefore sometimes considered a sixth stage of mitosis. All phases of mitosis, likewise as the flanking periods of interphase and cytokinesis before and after, are shown in Figure eight. Researchers' biochemical agreement of mitotic phases has profoundly increased in contempo years (Mitchison & Salmon, 2001), revealing that this highly orchestrated procedure involves hundreds, if not thousands, of cellular proteins.
Prophase
Mitosis begins with prophase, during which chromosomes recruit condensin and begin to undergo a condensation process that will continue until metaphase. In nigh species, cohesin is largely removed from the arms of the sis chromatids during prophase, allowing the private sis chromatids to be resolved. Cohesin is retained, notwithstanding, at the nearly constricted part of the chromosome, the centromere (Figure ix). During prophase, the spindle also begins to course as the two pairs of centrioles motion to opposite poles and microtubules begin to polymerize from the duplicated centrosomes.
Prometaphase
Prometaphase begins with the abrupt fragmentation of the nuclear envelope into many pocket-size vesicles that will eventually be divided between the future daughter cells. The breakup of the nuclear membrane is an essential footstep for spindle associates. Because the centrosomes are located exterior the nucleus in fauna cells, the microtubules of the developing spindle do not accept access to the chromosomes until the nuclear membrane breaks apart.
Prometaphase is an extremely dynamic role of the cell cycle. Microtubules apace get together and disassemble as they grow out of the centrosomes, seeking out attachment sites at chromosome kinetochores, which are circuitous platelike structures that assemble during prometaphase on one face of each sister chromatid at its centromere. As prometaphase ensues, chromosomes are pulled and tugged in opposite directions by microtubules growing out from both poles of the spindle, until the pole-directed forces are finally balanced. Sister chromatids do not break apart during this tug-of-state of war considering they are firmly attached to each other by the cohesin remaining at their centromeres. At the end of prometaphase, chromosomes have a bi-orientation, meaning that the kinetochores on sister chromatids are continued by microtubules to opposite poles of the spindle.
Metaphase
Side by side, chromosomes assume their most compacted country during metaphase, when the centromeres of all the jail cell's chromosomes line upwards at the equator of the spindle. Metaphase is especially useful in cytogenetics, because chromosomes can be virtually hands visualized at this phase. Furthermore, cells can exist experimentally arrested at metaphase with mitotic poisons such equally colchicine. Video microscopy shows that chromosomes temporarily end moving during metaphase. A complex checkpoint machinery determines whether the spindle is properly assembled, and for the most part, merely cells with correctly assembled spindles enter anaphase.
Anaphase
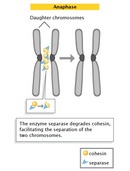
The progression of cells from metaphase into anaphase is marked by the abrupt separation of sister chromatids. A major reason for chromatid separation is the sharp degradation of the cohesin molecules joining the sis chromatids by the protease separase (Figure 10).
Two separate classes of movements occur during anaphase. During the showtime office of anaphase, the kinetochore microtubules shorten, and the chromosomes move toward the spindle poles. During the second part of anaphase, the spindle poles dissever as the non-kinetochore microtubules motion past each other. These latter movements are currently thought to be catalyzed past motor proteins that connect microtubules with reverse polarity so "walk" toward the cease of the microtubules.
Telophase and Cytokinesis
Mitosis ends with telophase, or the stage at which the chromosomes accomplish the poles. The nuclear membrane and so reforms, and the chromosomes brainstorm to decondense into their interphase conformations. Telophase is followed past cytokinesis, or the partition of the cytoplasm into two daughter cells. The daughter cells that result from this process have identical genetic compositions.
References and Recommended Reading
Cheeseman, I. M., & Desai, A. Molecular compages of the kinetochore-microtubule interface. Nature Reviews Molecular Cell Biology 9, 33–46 (2008) doi:10.1038/nrm2310 (link to article)
Cremer, T., & Cremer, C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nature Reviews Genetics 2, 292–301 (2001) doi:10.1038/35066075 (link to commodity)
Hagstrom, K. A., & Meyer, B. J. Condensin and cohesin: More than chromosome compactor and mucilage. Nature Reviews Genetics 4, 520–534 (2003) doi:10.1038/nrg1110 (link to article)
Hirano, T. At the heart of the chromosome: SMC proteins in activeness. Nature Reviews Molecular Cell Biological science 7, 311–322 (2002) doi:10.1038/nrm1909 (link to article)
Mitchison, T. J., & Salmon, E. D. Mitosis: A history of division. Nature Cell Biology 3, E17–E21 (2001) doi:x.1038/35050656 (link to commodity)
Paweletz, N. Walther Flemming: Pioneer of mitosis research. Nature Reviews Molecular Cell Biology 2, 72–75 (2001) doi:10.1038/35048077 (link to article)
Satzinger, H. Theodor and Marcella Boveri: Chromosomes and cytoplasm in heredity and development. Nature Reviews Genetics 9, 231–238 (2008) doi:x.1038.nrg2311 (link to commodity)
parramorehatevesserom.blogspot.com
Source: http://www.nature.com/scitable/topicpage/mitosis-and-cell-division-205